Recherchez sur le site !
Recherche avancée / SpécifiqueCatégories publications
+ Sciences De La Terre - Archéologie - Astronomie - Spéléologie - Ecologie - Pédologie - Volcanologie - L'hydrogéologie - Géomorphologie - Minéralogie - Pétrologie - Paléontologie - Géologie + Climatologie - Réchouffement climatique - Changement climatique + Plantes - Plantes Aromatiques - Plantes médicinales + Zoologie - Faunes + Botanique - Flors + Sciences humaines - Géo Eco Tourisme - L’anthropologie - L'Histoire - Démographie - Sociologie - Géographie - Patrimoine culturel
Géo éco tourisme inclusif

Géoparc et Recherche Scientifique
Le coins de l’étudiant



Blog Géoparc Jbel Bani
Environmental control of schistosomiasis through community participation in a Moroccan oasis (Géoparc Jbel Bani)
By Eline Boelee and Hammou Laamrani
International Water Management Institute, Addis Ababa, Ethiopia
Ministry of Education, Tabriquet, Sale´, Morocco
Summary
Akka oasis, in the province of Tata, southern Morocco, is one of the oldest foci of urinary schistosomiasis in Morocco where transmission is still taking place. We report the results of two studies: a cross-sectional snail survey investigated the distribution of Bulinus truncatus in relation to habitat factors in the Akka traditional irrigation system. The presence of aquatic vegetation, especially Potamogeton sp. was identified as a key factor determining snail occurrence and abundance in canals, impoundments and isolated small puddles and streamlets in the Akka riverbed. In a participatory rapid appraisal, the community identified snail control as a way to reduce transmission of schistosomiasis.
Without any further outside incentives, the local irrigation committee implemented repeated cleaning and vegetation removal in canals. A longitudinal study evaluated the effect of these measures on populations of B. truncatus. Snail and egg mass densities showed significant reductions after repeated vegetation clearing in the study sites. The participatory approach led to low-cost, environment-friendly schistosomiasis control measures that were effective and sustainable.
Introduction
In Morocco, transmission of urinary schistosomiasis occurs under a wide variety of climatic, geographical and geological conditions. In addition to natural habitats, transmission takes place in modern and traditional irrigation schemes, where the intermediate host Bulinus truncates can find many suitable breeding sites. The oasis of Akka is the most important focus of schistosomiasis in the province of Tata and was first described by Connet (1937). In 1982, the Moroccan Ministry of Health launched a national programme of schistosomiasis control (Laaziri & Benouna 1982) and snail control using molluscicides was carried out for several years in the province of Tata, combined with case detection and treatment.
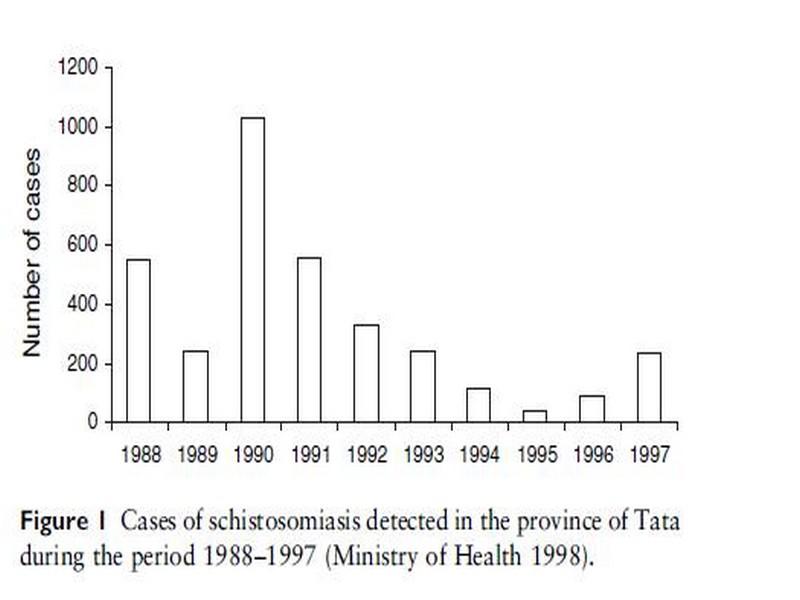
In a special campaign in 1995, mass treatment was applied in all exposed villages, where only pregnant women and children <2 years old were not treated. A total of 7759 people were treated with praziquantel (40 mg/kg) regardless of the infection status. Four months later, a case detection survey showed no cases. Ten months after treatment, however, cases were detected, indicating that transmission was continuing (Service des Maladies Parasitaires, Ministry of Health, unpublished data). Hence, Akka oasis is one of the few remaining foci in Morocco where transmission is still taking place. Half of the total number of cases of schistosomiasis detected in 1997 at the national level were found in the province of Tata, with the highest prevalence (1.5%) and incidence (8.5%) recorded in the country (Ministry of Health 1998). Figure 1 shows the annual number of schistosomiasis cases in the area over the period 1989–1997.
In 1997, the population exposed to schistosomiasis infection in the province of Tata was estimated to be 27 458. A total of 22 villages were exposed and 11 were affected. In the definition of the Ministry, exposure refers to individuals or communities living in areas where cases of schistosomiasis were detected in previous surveys or where ideal transmission conditions exist. It also includes villages with intermediate host snails, intense water contact, close to affected villages.
The present study aims to determine snail distribution in relation to habitat factors, and furthermore to evaluate the effect on B. truncates of repeated cleaning and vegetation removal. The contribution of local involvement in snail control to the schistosomiasis elimination programme launched by the Ministry of Health in 1994 is discussed.
Material and methods
The study area
The oasis of Akka is situated in province of Tata in the lower Draa basin in the far south of Morocco, 300 km from the city of Agadir. Agriculture depends almost entirely on a traditional irrigation scheme covering an irrigated area of approximately 1530 ha (Ministry of Agriculture 1996). The population in the palm oasis is estimated at 13 000 habitants. The region has a Saharan climate with cold and dry winters and dry and hot summers. The mean annual rainfall calculated over the period 1937–1990 did not exceed 81 mm/year. The mean monthly minimum temperature ranged from 5 to 25 C, the maximum from 20 to 44 C. The mean evaporation is estimated to be 2000 mm/year (Ministry of Agriculture, unpublished data).
The main crop in the oasis is the date palm. Under the palms, cereals and vegetables are grown in small garden plots to cover local needs around the year. The main source of income in Akka consists of money sent by relatives working as shopkeepers in Western and Central Morocco.
This migration constitutes a risk of extending schistosomiasis to regions where the disease now is nearly eradicated.
Other major health problems in the area are trachoma and conjunctivitis.
The irrigation system
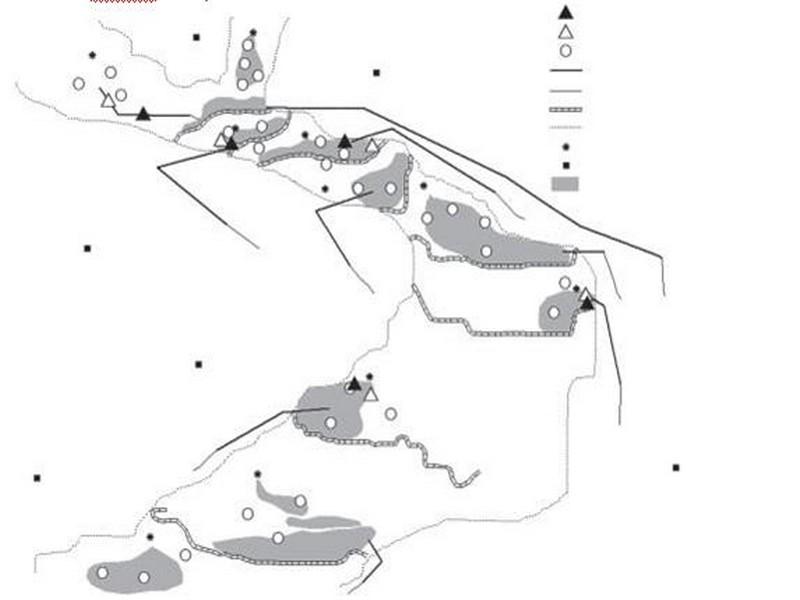
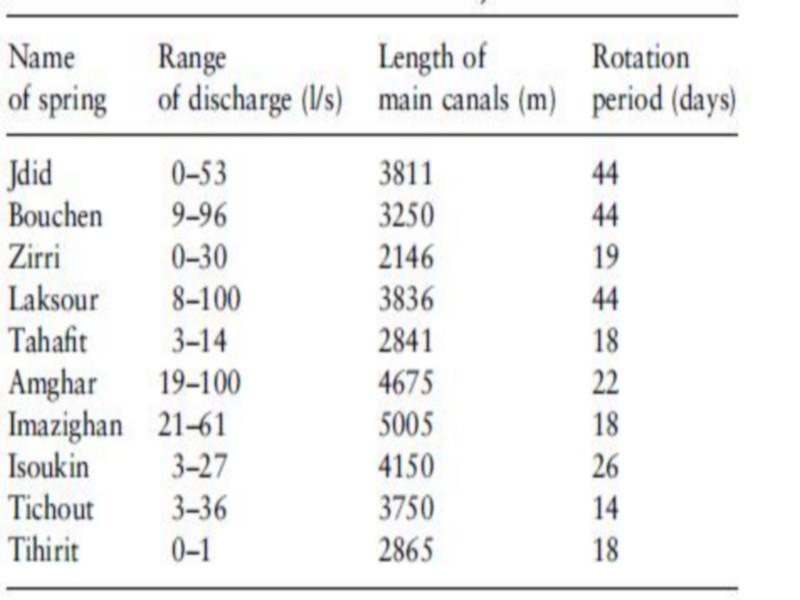
The irrigation system consists of 10 springs with varying discharges emerging in the Akka riverbed (Figure 2). Small cross-sectional dams have been constructed downstream of the springs to impound water. From these ponds the water is conveyed through a network of small canals to the fields.
The water from each impoundment is shared by a group of farmers who each are entitled to the entire flow for a certain period of time. The rotation period varies from 18 days in Tihirit spring to 44 days in Bouchen and Jdid (Table 1). This means that canals close to the riverbed convey water almost continuously, while further away, canals may be dry for several days or even weeks.
Of the 50 km total length of the irrigation system, 20 km have been replaced by cement canals in a rehabitation project in 1997, during the study period. Cleaning of the canals and impoundments is coordinated by a committee for each spring separately to ensure a maximum discharge. This activity is carried out periodically and consists of removing vegetation and silt. Such practices are common in most modern and rehabilitated irrigation systems in Morocco (Lahlou 1985).
Figure 2 Location of the sampling sites and selected intervention and control sites in the Akka riverbed.
Snail sampling method in these study two methods was combined: a drag scoop was used in deep water bodies whereas a quantitative sampling method with quadrates of 0.30 m2 was applied in shallow habitats. The number of sampled quadrates and their distance was defined with regard to the habitat surface and morphological variation. The minimum number of quadrates searched was fixed at three but up to five quadrates were sampled. All material such as boulders, gravel as well as floating, submerged and emerging vegetation was searched for snails and egg masses. After counting, all snails and egg masses were returned to their respective habitats. Density is expressed as number of snails, egg masses per m2.
Habitat characteristics
Based on preliminary results from 1996, the present survey focused on sites located in the bed of the Akka River. The fields canals further away from the riverbed are dry between irrigation turns and not suitable to Bulinus snails, so these were not considered in the present study.
Several ecological factors were measured at each sampling site. The depth of the water was measured using a calibrated stick. Dimensions of the water bodies were measured for small habitats and estimated visually for large sites. Dissolved oxygen, pH and conductivity were measured with metres at the water surface between 10 am and noon. Water flow velocity was estimated in sites with flowing water using a cork float and then classified in three categories: stagnant (0 m/s), moderate flow (0.01–0.1 m/s) and high flow (>0.10 m/s). Vegetation in each sampling site was sorted according to species and the percentage of cover was estimated in six classes of 0 < 5%, 6–25%, 26–50%, 51–75% and >75%. The type of substratum was categorized into clay/silt, sand, gravel, pebbles or bedrock. Other types of substratum were gathered in a separate category and included boulders, mud and organic material in different proportions.
Table 1 some characteristics of the springs providing water for the traditional irrigation systems in Akka oasis (Ministry of Agriculture 1996, unpublished data; based on 12 measurements taken between 1939 and 1993)
Data analysis
Chi-square tests were used to compare the frequency of occurrence of B. truncatus snails and egg masses in different habitats. In order to avoid low cell counts, habitats were grouped into three groups according to flow velocity categories. Snail and egg mass densities [log10 (individuals or egg masses/m2 + 1)] were compared using one-way anova. The relationship between occurrence of B. truncates and habitat factors was determined using logistic regression (Hosmer & Lemeshow 1989), corrected for the habitat. Spearman rank correlation with two-tailed test of significance was used to determine associations between densities of B. truncates and habitat factors.
Snail control
In a participatory rapid appraisal in Akka in May 1997, the community identified several intervention strategies for schistosomiasis control. These included measures to reduce water contact, snail control interventions, health education and improvements to the health centre (Boelee 1999).
Within 1 year after formulation of the recommendations, a doctor had been appointed to the health post, collaboration between the Village Committees and Spring Committees was reinforced and weeds were removed from transmission sites. Normally, canals are cleaned once a year to improve the conveyance efficiency. Motivated by the discussions on schistosomiasis, the farmers took the initiative to clean the canals twice between May and October 1997 and again three times between October 1997 and March 1998. This intensified canal cleaning consisted of removal of vegetation and silt, creating a smoother canal bed and steeper banks, allowing for higher water flow velocities.
Eight intervention sites were sampled whereas eight others were used for control. However, ongoing rehabilitation works repeatedly perturbed four study sites while two other sites at Tichout spring were dry at the second sampling. Thereafter, two groups of five sampling sites each were considered in the follow-up (Figure 2).
These sites were sampled twice before the implementation in October and May 1996 and twice afterwards in October 1997 and March 1998. Snail and egg mass densities were estimated using the above-mentioned quantitative method.
Results
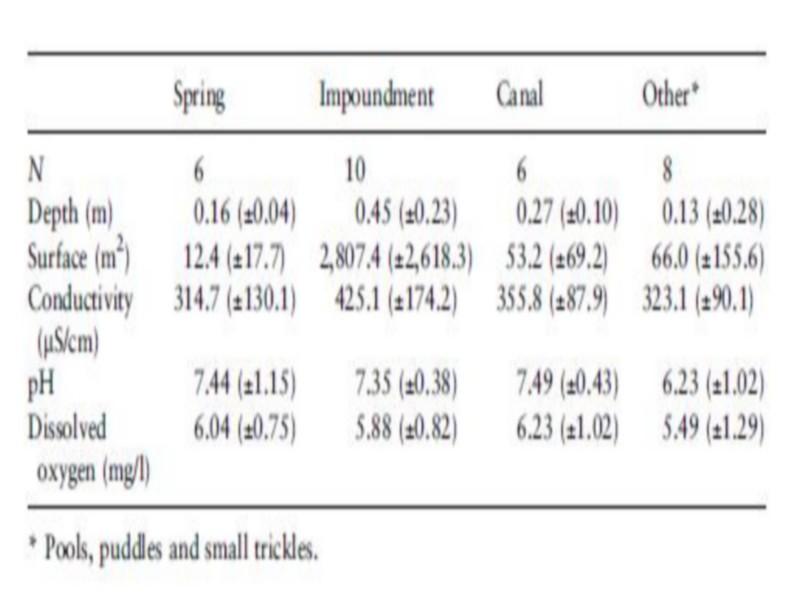
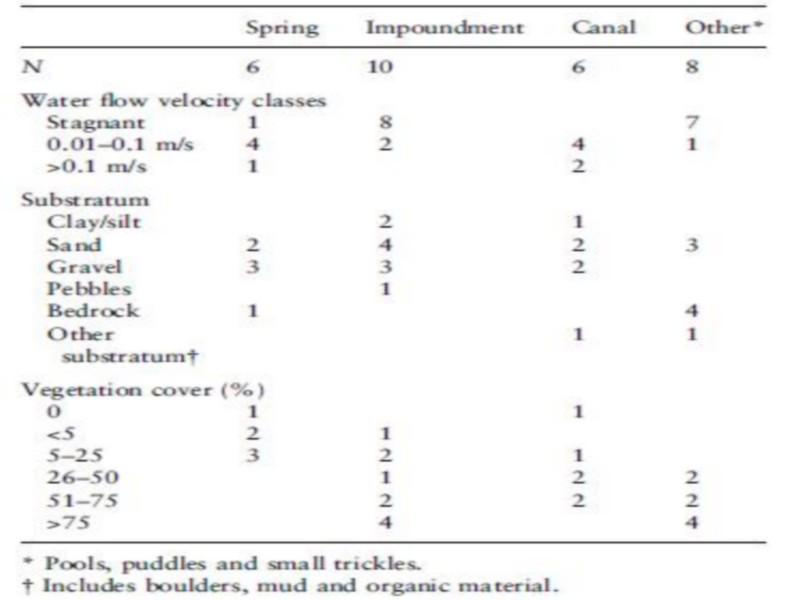
The 30 sampled sites consisted of springs, impoundments and pools, lined and unlined canals, puddles, marshes created by seepage, and isolated small trickles. The open water surface varied significantly between habitats, but did not significantly affect snail occurrence and density. The impoundments were the largest and deepest habitats, although few impoundments had a depth of more than 0.5 m (Table 2). The different categories of water flow velocity corresponded with the type of habitat (Table 3). Stagnant water was found in impoundments, pools and puddles.
Trickles had slowly flowing water, while moderate-to-fast flows were observed in springs and canals. Most human activities take place at the impoundments, which constitute popular swimming and fishing ponds for children and suitable places for laundry and other activities by women.
Substratum composition varied between sites from bare rock with organic debris to sediments of mineral and/or organic origin (Table 3). Sand and gravel were the most frequent types. Pebbles and gravel were found in canals and at the banks of some impoundments. Sand was found in springs, impoundments and some sections of the riverbed. Occasionally more than two types of substratum in the same sampling point were found.
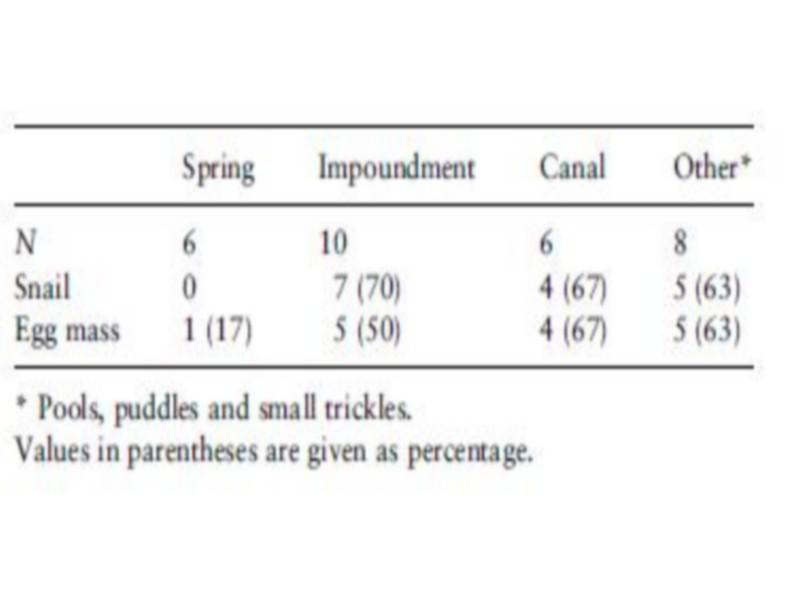
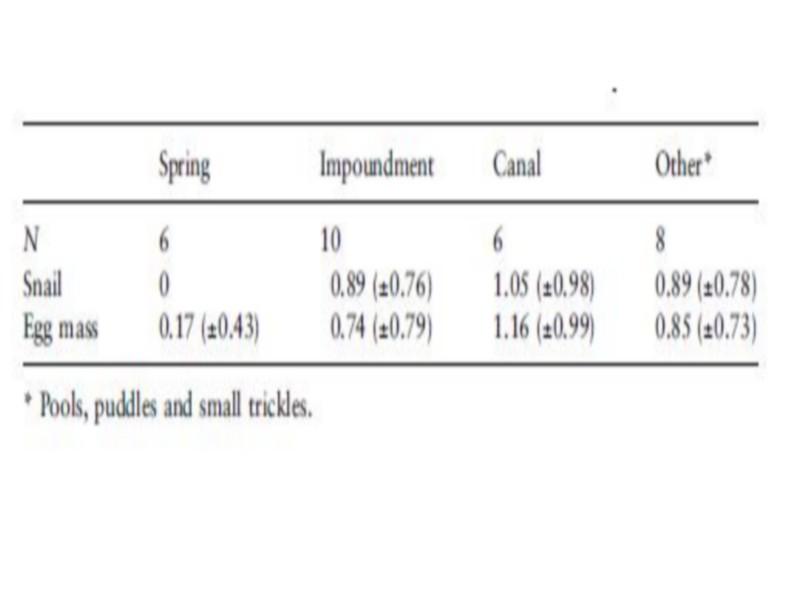
Occurrence of B. truncates snails (Table 4) differed significantly between habitats (v2 ¼ 8.57, P < 0.05). Adult B. truncates were not found in the sampled springs, although egg masses were collected at one of the six sites.
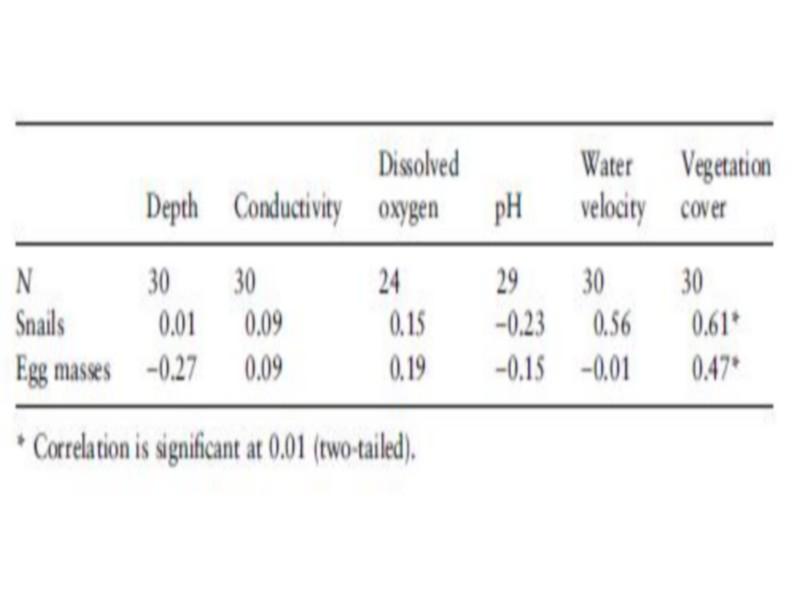
The highest frequency of occurrence of B. truncates was noticed in deep impoundments (Table 4), whereas the highest density was found in unlined canals (Table 5). Snail densities differed significantly between habitats (F ¼ 4.05, P < 0.05). The highest snail and egg mass densities were recorded in protected pockets of unlined canals and small trickles, while the lowest egg mass density was found in springs. Although B. truncates was apparently associated with shallow habitats, there was no significant association between depth and snail density (Table 6). Contrarily, a negative (r ¼) 0.27, but not significant) association was found between egg mass density and depth, which could indicate the predilection of B. truncates for shallow habitats.
Highest water flow velocities were recorded at springs and at the upstream part of canals conveying water from impoundments to the fields. However, in canals with relatively rapid flow, areas with slow flowing water were found as well. The highest snail density of B. truncates was recorded in some of these protected areas. This could partly explain the positive (r ¼ 0.56, but not significant) association between water velocity and snail density (Table 6).
Aquatic vegetation consisted of Potamogeton sp., Juncus maritimus, Chara vulgaris, Spirogyra sp., Rubbia sp. And Myriophyllum sp. Potamogeton sp. was the most frequent, occurring in 22 sites of 30 sampled. Decaying palm leaves as well as leaves of Cucurbita sp. and Fragmites communis were found in several sites. Bulinus truncatus snails and egg masses were frequently attached to them. Palm leaves were frequent in snail habitats, especially in October 1996 and 1997, which is the season for date harvesting. The distribution of vegetation cover over the sampled sites is presented in Table 3. Macroscopic vegetation was absent in only two sites, whereas 14 of the sites were more than 50% covered with vegetation. A few sites were totally Table 2 Average values (±SD) for physical and chemical characteristics in different habitats in Akka oasis, southern Morocco, in May 1997
Table 3 Frequency of classes of water flow velocity, vegetation cover and types of substratum in different habitats in Akka oasis, southern Morocco, in May 1997
Table 4 Frequency of occurrence (as percentage) of Bulinus truncatus snails and egg masses in different habitats in Akka oasis, southern Morocco, in May 1997
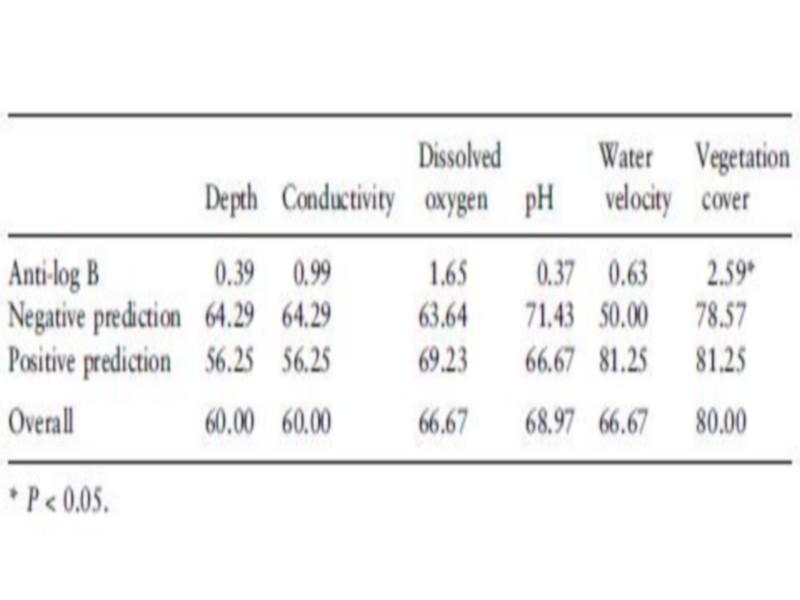
Covered due to abundant aquatic vegetation with mainly Potamogeton sp. Density and occurrence of B. truncates were significantly associated with the vegetation cover (r ¼ 0.61, P < 0.01, Tables 6 and 7).
Table 5 Mean density [log10 (count + 1)] (±SD) of Bulinus truncatus snails and egg masses in different habitats in Akka oasis, southern Morocco, in May 1997
Table 6 Correlation between density of Bulinus truncatus snails and egg masses and habitat parameters using Spearman rank correlation
Table 7 Association between occurrence of Bulinus truncatus and habitat parameters using logistic regression after correction for habitat
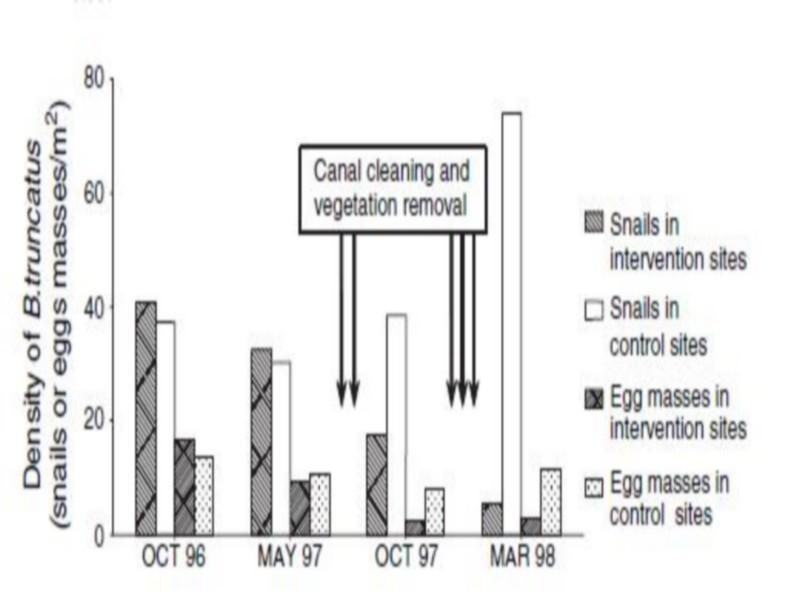
Figure 3 Density of Bulinus truncates snails (snails/m2) and eggs (egg masses/m2) in intervention and control sites in Akka riverbed (the arrows indicate the approximate timing of interventions). Intense activity of the human population at the springs, which may affect the occurrence of aquatic plants.
Physico-chemical factors studied were within the tolerance limits of Bulinus sp. (Watson 1958; Appleton 1978; Brown 1994). Slowly flowing water might be a preferred environment for B. truncatus because of the better aeration, more stable temperature and wash out of dissolved contents and unfavorable putrefactive products (Mousa & El Hassan 1972).
Abundance of aquatic plants is a key factor for the occurrence and density of B. truncatus in the study area.
Potamogeton sp. was the most frequent and abundant type of aquatic vegetation and B. truncatus and egg masses were frequently found attached to its leaves. Indeed, several studies have shown that aquatic vegetation is a factor of outstanding importance in snail distribution and abundance.
An association between B. truncatus and aquatic vegetation (Potamogeton sp.) was also reported by Tsafack (1997) in the Ladgo irrigation scheme in Cameroon. In the Gezira irrigation scheme in Sudan, Hilali et al. (1985) found that snails were often associated with aquatic macrophytes. A similar association was observed by Madsen et al. (1988).
Aquatic vegetation makes a habitat more attractive for B. truncates for several reasons: it provides shelter from high water flow velocities and excessive sunlight, it offers support for egg masses and it constitutes the ideal substratum for the algae that constitute the snail’s main food. In our study, vegetation removal and cleaning of irrigation canals led to a substantial reduction in density of B. truncates snails and their egg masses. The effect of this control measure on snails was probably due to a combination of the direct removal of B. truncatus and its egg masses attached to vegetation, the reduction of plant food resources, and the impact of increased water flow velocity.
Silt removal creates a synergetic effect as it renders snail habitats unsuitable for aquatic vegetation, which in turn affects availability of food and shelter for snails (Thomas & Tait 1984). Canal cleaning and sediment removal reduce resistance and increase water flow velocity (Oomen ET al.1990). Increased water flow velocities hinder snail movement and development directly but also reduce the availability of food resources, thus reducing snail populations indirectly.
Vegetation removal and cleaning have been applied under different conditions in Morocco and elsewhere to control schistosomiasis intermediate hosts. In an intervention study in Central Morocco the authors compared three different environmental snail control measures that removed snails, egg masses, aquatic plants and silt (Laamrani & Boelee 2002). Intensified cleaning of hydraulic structures led to a short-term reduction in density of B. truncates but the intervention could not be sustained by the farmers in this area and snail populations rapidly re-colonized the habitats (Laamrani et al. 2000). In the Rahad irrigation scheme in the Sudan, Meyer-Lassen (1992) observed the disappearance of B. truncatus after the removal of aquatic plants. In contrast, Hilali et al. (1985) reported less impressive results from the Gezira irrigation scheme, where mechanical weed removal alone did not lead to a significant reduction of the snail population, because of rapid re-colonization of the canals by weeds.
The intervention tested in Akka was not only successful in reducing snail populations, but had a potential impact on transmission of schistosomiasis as well, as increased water flow velocities hamper the penetration of the skin by Schistosoma cercariae. The control measure of cleaning canals was thus effective in the short-term and possibly in the long-term as well, as the intervention has been repeated several times. Contrary to other studies, here the intervention was initiated, planned, implemented and continued by the community itself, as an alternative to existing chemical snail control techniques applied by the Ministry of Health. As such, it can be considered a sustainable method of schistosomiasis control. It is also environment friendly as it reduces the need for application of harmful chemical molluscicides.
The intensified cleaning also improved canal conveyance efficiencies: with higher water flow velocities, water losses in the earthen canals were reduced significantly. As a result, a larger flow reaches the end of the canals, which is easier for the farmers to manipulate onto their fields.
Another interesting outcome of the study was the suggestion by the community to integrate schistosomiasis and trachoma control. Such a combined approach could have far reaching implications for improving the health status of the inhabitants of Akka oasis.
The approach of involving the population in an early stage of problem definition and identification of counter measures can be recommended for disease control in other situations as well (Laamrani et al. 2001). It will help to develop locally appropriate measures for disease control. For schistosomiasis, environmental snail and transmission control are welcome additions to case detection and treatment-based approaches. As mentioned by several authors, no single control strategy has proven effective on its own in the control of schistosomiasis (Thomas 1987; Madsen & Christensen 1992; World Health Organization 1998; Sturrock 2001). An integrated approach, involving the application of several measures simultaneously, is needed to control schistosomiasis.
Especially in the Moroccan context of schistosomiasis elimination, snail control is imperative to prevent re-infection, while community-based environmental measures also contribute to reducing the cost per positive case.
In conclusion, our findings indicate that local participation in environmental schistosomiasis control is feasible. The implemented measures were initiated, repeatedly applied and paid for by the community. Costs were low and benefits surpassed schistosomiasis control by increasing irrigation performance and reducing the application of pesticides in a vulnerable environment.
The local population is keen to participate in schistosomiasis control when the benefit of the contribution is clear to them and the actions to be taken are defined by themselves, in a participatory rather than a top–down approach.
Acknowledgements
Authors thank the health authorities at the Province Me´dicale, SIAAP of Tata and the Centre de Sante´ of Akka. Thanks are due to the staff of the Service des Maladies Parasitaires for their assistance in this study. Dr H. Laamrani is grateful to the Netherlands Foundation for the Advancement of Tropical Research (WOTRO, Grant no. WB 93-260), the Danish Bilharziasis Laboratory and the Danish International Development Assistance (Danida). Dr E. Boelee appreciates the support of the Department of Parasitology at Leiden University Medical Center, as well as the Irrigation and Water Engineering Group at Wageningen University, both in the Netherlands. Parts of the study were funded under the EC/Avicenne project CT93-AVI-0004 ‘Environmental control of schistosomiasis in irrigation schemes of the Mediterranean regions’. In addition, authors would like to thank Henry Madsen at the Danish Bilharziasis Laboratory for his help in the statistical analysis and Bruno Gryseels at the Institute for Tropical Medicine Prince Leopold for his general support and encouragement.
References
Appleton CC (1978) Review of literature on abiotic factors influencing the distribution and life cycles of bilharziasis intermediate host snails. Malacological Review 11, 1–25.
Boelee E (1999) Irrigation ecology of schistosomiasis: environmental control options in Morocco. PhD thesis, Wageningen University, the Netherlands.
Brown D (1994) Freshwater Snails of Africa and their Medical Importance. 2nd edn. Taylor and Francis, London.
Connet M (1937) Existence d’un foyer de bilharziose ve´sicale a` Akka (de´couvert pour la premie`re fois). Maroc Me´dical 63, 365–366.
Hilali AMH, Dessouqui LA, Wassila M, Daffalla AA&Fenwick A (1985) Snails and aquatic vegetation in Gezira irrigation canals. Journal of Tropical Medicine and Hygiene 88, 75–81.
Hosmer DW & Lemeshow S (eds). (1989) Applied Logistic Regression. John Wiley & Sons, New York.
Laamrani H & Boelee E (2002) Roˆ le des parame`tres de conception, de gestion et de maintenance des pe´rime`tres irrigue´s dans la transmission et la lutte contre la bilharziose au Maroc central. Cahiers Agriculture 11, 23–29.
Laamrani H, Khallaayoune K, Boelee E, Laghroubi MM, Madsen H & Gryseels B (2000) Evaluation of environmental methods to control snails in an irrigation system in Central Morocco. Tropical Medicine and International Health 5, 545–552.
Laamrani H, Madsen H & Boelee E (2001) Snail control in Africa: towards a community-based environmental control. In: Proceedings of ‘Workshop on Medical and Veterinary Malacology in Africa’, Harare, Zimbabwe, November 8–12, 1999, Harare, Zimbabwe (eds H Madsen, CC Appleton & M Chimbari).
Danish Bilharziasis Laboratory, Charlottenlund, pp. 183–192.
Laaziri M & Benouna M (1982) Guide de la lutte contre la bilharziose. Direction des Affaires Techniques, Ministe`re de la Sante´ Publique, Rabat, Morocco.
Lahlou O (1985) La gestion des re´seaux dans certains grands pe´rime` tres irrigue´s marocains. Hommes, Terre et Eaux 59, 97–103.
Madsen H & Christensen NØ (1992) Intermediate hosts of schistosomes: ecology and control. Bulletin of the Society for Vector Ecology 17, 2–9.
Madsen H, Daffalla AA, Karoum KO & Frandsen F (1988) Distribution of freshwater snails in irrigation schemes in the Sudan. Journal of Applied Ecology 25, 853–866.
Meyer-Lassen J (1992) Studies on snail populations in the Rahad agricultural scheme, the Sudan, with special emphasis on Bulinus truncatus (Audouin) and Biomphalaria pfeifferi (Kraus) (Gastropoda: Planorbidae), snail intermediate hosts for species of the genus Schistosoma. PhD thesis, University of Copenhagen, Denmark.
Ministry of Agriculture (1996) Second projet de de´veloppement de la petite et moyenne hydraulique (4eme tranche). Pe´rime`tre d’Akka. Administration du Genie Rural, Direction d’Ame´nagements Hydro-Agricoles, Rabat, Morocco.
Ministry of Health (1998) Etat d’avancement des programmes de lutte contre les maladies parasitaires. Anne´es 1996 and 1997.
Service des Maladies Parasitaires, Direction de l’Epide´miologie et de Lutte Contre les Maladies, Ministe`re de la Sante´, Rabat, Morocco.
Mousa AH & El Hassan AAA (1972) The effect of water movements on the snail intermediate hosts of schistosomiasis in Egypt. Journal of the Egyptian Medical Association 55, 255–260.
Oomen JMV, de Wolf J & Jobin WR (1990) Health and Irrigation.
Incorporation of Disease Control Measures in Irrigation, a Multi- faceted Task in Design, Construction, Operation. International Institute for Land Reclamation and Improvement/ILRI Publication 45, Wageningen.
Sturrock RF (2001) Schistosomiasis epidemiology and control: how did we get here and where should we go? Memorias do Instituto Oswaldo Cruz 96(Suppl.), 17–27.
Thomas JD (1987) A holistic view of schistosomiasis and snail control. Memo´ rias do Instituto Oswaldo Cruz, Rio de Janeiro 82, 183–192.
Thomas JD & Tait AI (1984) Control of snails hosts of schistosomiasis by environmental manipulation: a field and laboratory appraisal in the Ibadan area, Nigeria. Philosophical Transactions of the Royal Society, London B305, 201–253.
Tsafack F (1997) Ecology of schistosome intermediate hosts in the Benue Upper-Valley area. PhD thesis, University of Copenhagen, Denmark.
Watson JM (1958) Ecology and distribution of Bulinus truncates in the Middle East: with comments on the effects of some human activities in their relationship to the snail host on incidence of bilharziasis haematobia in the Middle East and Africa. Bulletin of the World Health Organization 18, 833–894.
World Health Organization (WHO) (1998) Report of the WHO Informal Consultation on Schistosomiasis Control. Geneva, 2–4 December 1998. WHO/CDS/CPC/SIP/99.2. Communicable Diseases Prevention and Control, World Health Organization, Geneva.
Authors
Eline Boelee, International Water Management Institute, PO Box 5689, Addis Ababa, Ethiopia. Tel.: +251-1-457222;
Fax: +251-1-461252; E-mail: e.boelee@cgiar.org (corresponding author).
Hammou Laamrani, Ministry of Education, BP 881, Tabriquet, Sale´, Morocco. E-mail: laamrani2001@yahoo.co.uk
Source web: Prof. Eline Boelee and Prof. Hammou Laamrani
Les tags en relation
Dictionnaire scientifique
Plus de 123.000 mots scientifiques
Les publications
Géo parc Jbel Bani

Circuits & excursions touristiques
cartothéques


Photothéques
Publications & éditions